Top 5 Pharma
Accelerating new medicine time-to-market through a state-of-the-art clinical data analytics solution
Case study
At a glance
We worked with clinical analytics specialists to design, test and build a cloud-based, user-centered Statistical Computing Environment (SCE), drastically reducing drug time-to-market and enhancing global collaboration.
Client
Top 10 Pharma
Sector
Health & Life Sciences
Project
Statistical Computing Environment
Activities
Stakeholder interviews
User interviews
Design Thinking workshops
Personas
User journeys
Functional & technical workshops
Clickable prototype
Usability testing (multiple rounds)
Graphic design
Design system
Cloud & systems architecture
Development in Agile sprints
Quality testing, including UXQA
Technologies
AWS Cloud
Single Sign On
Microservices Architecture
Lustre File System
Autoscaling Kubernetes Containers
Java backend
React/Redux frontend
SAS Grid
SAS Studio
R Cluster
R Studio
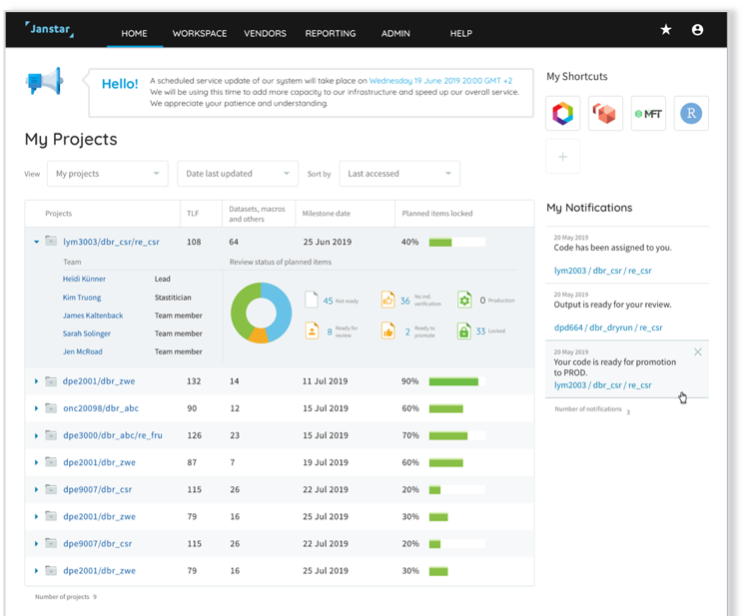
Modernizing clinical data analytics for R&D
Our client's legacy system was reaching its "end of life" and needed to be redesigned to suit new ways of working and new technologies such as cloud computing and open-source statistical programming languages like R. The company was keen to ensure that the new system met modern-day standards of performance, modularity, and usability.
Whitespace joined clinical analytics specialists from d-wise to design and build a world-class enterprise solution in record time, compared to similar efforts ongoing at other Pharma companies.
User-centered design for a successful outcome
Whitespace guided the client stakeholders and internationally distributed development teams through a complete user-centered design approach.
We led multiple UX workshops in the USA and Europe, as well as conducted interviews with statisticians, medical writers, data analysts, and statistical programmers in North America, Europe, and Asia.
Using the insights obtained from the UX research phase, we recommended a new optimized workflow and user interface design for a cloud-based statistical analysis & reporting solution that we then tested with real users from the client's globally distributed team.
The custom build is based on a React/Redux front-end, a Java back-end, and SAS/R grids deployed on Amazon EKS.
A 30-person delivery team, of which Whitespace was (and still is) an integral part, collaborated across 9 countries and multiple time zones to deliver this project on time and on budget. The project continues to this day as enhancements are added in an iterative, user-centered approach.

We really enjoyed working with you and everyone from Whitespace. Your experience, knowledge, and professionalism were truly something we needed on the project to make it a success. And your self-motivation, enthusiasm, dedication, and diligence are a true inspiration! Big kudos to all of you and a big thank you for everything.
Director, Scientific Computing Operations, Statistics & Decisions Sciences
What success looks like
Today, our client's bespoke SCE solution is used by over 800 statistical programmers and clinical data analytics professionals worldwide to accelerate the delivery of new medicines and vaccines to market.
The new product is widely considered to be one of the leading SCE platforms currently in use in the pharmaceutical industry and is the envy of the company's peers. The combination of a scalable cloud computing architecture with a modern user interface, clear workflows, robust audit trail, and an open-source programming environment is leading to dramatic efficiency gains at the company.

800+
Members of R&D impacted
2x
Development timeframe
50%
Lower cost than alternative systems
1.5x
Faster time to market for drugs
Julia Borkenhagen
Chief Experience Officer
It's not easy to understand the complexities of the clinical trial process, much less the nuances of data analytics within the biostats function of a very large pharmaceutical company. We learned so much during this project and it has enabled us to expand our UX, business analysis, and software development practices into the clinical space.
Like what you see?